Polarity, Resonance, And Electron Pushing: Crash Course Organic Chemistry #10:
Polarity, Resonance, and Electron Pushing: Crash Course Organic Chemistry #10:
We’ve all heard the phrase “opposites attract.” It may or may not be true for people, but it’s definitely true in organic chemistry. In this episode of Crash Course Organic Chemistry, we’re learning about electronegativity, polarity, resonance structures, and resonance hybrids. We’ll practice a very important skill for this course that will help us avoid a lot of memorization in the future: electron pushing. It’ll be a lot of trial and error at first, but we all start somewhere!
More Posts from Amateurchemstudent and Others
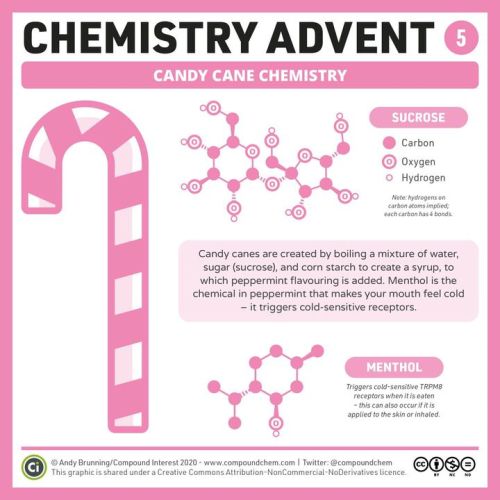
It’s day 5 of #ChemAdvent – here’s why peppermint candy canes make your mouth feel cold! bit.ly/chemadvent2020 https://ift.tt/2JM6bZ7
The Name’s Bond ... Ionic Bond.
This is the first in my short series of the three main types of bond - ionic, metallic and covalent. In this, you’ll learn about the properties of the compounds, which atoms they’re found between and how the bonds are formed. Enjoy!
When electrons are transferred from a metal to a non-metal, an ionic compound is formed. Metals usually lose electrons and non-metals usually gain them to get to a noble gas configuration. Transition metals do not always achieve this.
Charged particles that have either lost or gained electrons are called ions and are no longer neutral - metal atoms lose electrons to become positive ions (cations) whereas non-metals gain electrons to become negative ions (anions).
The formation of these ions is usually shown using electron configurations. Make sure you know that the transfer of electrons is not the bond but how the ions are formed.
An ionic bond is the electrostatic attraction between oppositely charged ions.
You need to know how to explain how atoms react with other atoms and for this the electron configurations are needed. You can use dot and cross diagrams for this.

Ionic solids hold ions in 3D structures called ionic lattices. A lattice is a repeating 3D pattern in a crystalline solid. For example, NaCl has a 6:6 arrangement - each Na+ ion is surrounded by 6 Cl- and vice versa.
Ionic solids have many strong electrostatic attractions between their ions. The crystalline shape can be decrepitated (cracked) on heating. Ionic Lattices have high melting and boiling points since they need more energy to break because atoms are held together by lots of strong electrostatic attractions between positive and negative ions. The boiling point of an ionic compound depends on the size of the atomic radius and the charge of the ion. The smaller the ion and the higher the charge, the stronger attraction.

Look at this diagram. It shows how atomic radius decreases across a period regularly. This is not the case with the ions. Positive ions are usually smaller than the atoms they came from because metal atoms lose electrons meaning the nuclear charge increases which draws the electrons closer to the nucleus. For negative ions, they become larger because repulsion between electrons moves them further away - nuclear charge also decreases as more electrons to the same number of protons.
Ionic substances can conduct electricity through the movement of charged particles when molten or dissolved (aqueous). This is because when they are like this, electrons are free to move and carry a charge. Ionic solids cannot conduct electricity.

Ionic compounds are usually soluble in water. This is because the polar water molecules cluster around ions which have broken off the lattice and so separate them from each other. Some substances like aluminium oxide have too strong electrostatic attractions so water cannot break up the lattice - it is insoluble in water.
Molecular ions such as sulfate, nitrate, ammonium or carbonate can exist within ionic compounds. These compounds may have covalent bonds within the ions but overall they are ionic and exhibit thee properties described above.
SUMMARY
When electrons are transferred from a metal to a non-metal, an ionic compound is formed.
Charged particles that have either lost or gained electrons are called ions and are no longer neutral - metal atoms lose electrons to become positive ions (cations) whereas non-metals gain electrons to become negative ions (anions).
The formation of these ions is usually shown using electron configurations. The transfer of electrons is not the bond but how the ions are formed.
An ionic bond is the electrostatic attraction between oppositely charged ions.
Ionic solids hold ions in 3D structures called ionic lattices. A lattice is a repeating 3D pattern in a crystalline solid.
Ionic solids have many strong electrostatic attractions between their ions. The crystalline shape can be decrepitated (cracked) on heating.
Ionic Lattices have high melting and boiling points since they need more energy to break because atoms are held together by lots of strong electrostatic attractions between positive and negative ions.
The boiling point of an ionic compound depends on the size of the atomic radius and the charge of the ion. The smaller the ion and the higher the charge, the stronger attraction.
Positive ions are usually smaller than the atoms they came from because metal atoms lose electrons meaning the nuclear charge increases which draws the electrons closer to the nucleus. Negative ions become larger because repulsion between electrons moves them further away - nuclear charge also decreases as more electrons to the same number of protons.
Ionic substances can conduct electricity through the movement of charged particles when molten or dissolved (aqueous). This is because when they are like this, electrons are free to move and carry a charge. Ionic solids cannot conduct electricity.
Ionic compounds are usually soluble in water because the polar water molecules cluster around ions which have broken off the lattice and so separate them from each other.
Some substances like aluminium oxide have too strong electrostatic attractions so water cannot break up the lattice - it is insoluble in water.
Molecular ions such as sulfate, nitrate, ammonium or carbonate can exist within ionic compounds. These compounds may have covalent bonds within the ions but overall they are ionic and exhibit thee properties described above.
Nomenclature - what in the organic chemistry is it?
Organic chemistry is so widely studied it requires a standard system for naming compounds, developed by IUPAC. Nomenclature is simply naming these organic compounds.
So, you want to be an organic chemist? Well, it starts here. Are you ready?
(psst… once you’ve learnt this theory, try a quiz here!)
1. Count your longest continuous chain of carbons.
Bear in mind that some chains may be bent. You’re looking for the longest chain of subsequent carbon atoms. This number correlates to root names that indicate the carbon chain length, listed below:

The second part of naming your base comes from the bonding in the chain. Is it purely single bonds or are there double bonds in there? If you are familiar with carbon chemistry, you’ll know that saturated hydrocarbons are called alkanes and unsaturated hydrocarbons are called alkenes. Therefore, the syllable -ane is used when it has only single bonds and the syllable -ene is used when it has some double bonds. For example:
Sometimes carbon chains exist in rings rather than chains. These have the prefix of -cyclo.
2. Identify your side chains attached to this main carbon and name them.
Side chains are added as prefixes to the root names. Sometimes called substituents, these are basically anything that comes off the carbon chain. Examples of the prefixes are listed below:

There are other prefixes such as fluoro (-F) and chloro (-Cl) which can describe what is coming off the chain.
3. Identify where each side chain is attached and indicate the position by adding a number to the name.
We aim to have numbers as small as possible. For example, if bromine is on the second carbon of a 5-carbon saturated chain, we number it as 2-bromopentane instead of 4-bromopentane, since it would essentially be 2-bromopentane if it was flipped. Locant is the term used for the number which describes the position of the substitute group, e.g. the ‘2′ in 2-chlorobutane is the locant.
Sometimes there are two or more side chains e.g. a methyl group and a chlorine attached to a pentane. In these cases, these rules apply:
1. Names are written alphabetically.
2. A separate number is needed for each side chain or group.
3. Hyphens are used to separate numbers and letters.

This would be named 2-chloro-3-methyl-pentane. This is because the longest chain of carbons is 5 (pentane), the chlorine is on the second carbon (2-chloro) and the methyl group is on the third carbon (3-methyl). It is 2-chloro rather than 4-chloro as we aim to have as small as numbers as possible.
Another variation of this step to be aware of is how many of the same side chains or groups there are, for example, having two methyl groups would be dimethyl rather than solely methyl. Each group must also be given numbers separated by commas to show where each one is located.
The list of these prefixes is found here:

Convention does not usually require mono- to go before a single group or side chain.
4. Number the positions of double bonds if applicable.
Alkenes and other compounds have double bonds. These must be indicated with numbers. For example, pent-2-ene shows that the double bond is between carbon 2 and carbon 3. The number goes in the middle of the original root name e.g. butene, pentene.
(!) Below is a list of functional groups that you may need to study for the AS and A Level chemistry exams. “R” represents misc. carbons. It is important to know that some groups are more prioritised than naming. From the most to least priority: carboxylic acid, ester, acyl chloride, nitrile, aldehyde, ketone, alcohol, amine, alkene, halogenalkane. It is worthwhile learning these.

bigger version here (I suggest downloading and printing it)
But wait, there’s more:
Here are some things to bear in mind when naming organic compounds:
1. The letter ‘e’ is removed when there are two vowels together e.g. propanone rather than propaneone. The ‘e’ isn’t removed when it is next to consonant, e.g. propanenitrile isn’t propannitrile.
2. When compounds contain two different, one is named as part of the unbranched chain and the other is named as a substituent. Which way round this goes depends on the priority.
SUMMARY
Count your longest continuous chain of carbons.
Chains may be bent. You’re looking for the longest chain of subsequent carbon atoms. This number correlates to root names that indicate the carbon chain length, e.g. pentane.
The second part of naming your base comes from the bonding in the chain. Is it purely single bonds or are there double bonds in there? The syllable -ane is used when it has only single bonds and the syllable -ene is used when it has some double bonds.
Rings have the prefix of -cyclo.
Identify your side chains attached to this main carbon and name them.
Side chains are added as prefixes to the root names. Sometimes called substituents, these are basically anything that comes off the carbon chain.
There are other prefixes such as fluoro (-F) and chloro (-Cl) which can describe what is coming off the chain.
Identify where each side chain is attached and indicate the position by adding a number to the name.
We aim to have numbers as small as possible. Locant is the term used for the number which describes the position of the substitute group, e.g. the ‘2′ in 2-chlorobutane is the locant.
Sometimes there are two or more side chains e.g. a methyl group and a chlorine attached to a pentane. In these cases, names are written alphabetically, a separate number is needed for each side chain or group and hyphens are used to separate numbers and letters.
When there are two or more of the same side chains or substituent groups, these must also be given numbers separated by commas to show where each one is located.
Number the positions of double bonds if applicable.
Alkenes and other compounds have double bonds. These must be indicated with numbers. The number goes in the middle of the original root name e.g. butene, pentene.
It is worthwhile learning the other functional groups that can be added on.They have varying priorities.
The letter ‘e’ is removed when there are two vowels together e.g. propanone rather than propaneone. The ‘e’ isn’t removed when it is next to consonant, e.g. propanenitrile isn’t propannitrile.
When compounds contain two different, one is named as part of the unbranched chain and the other is named as a substituent. Which way round this goes depends on the priority.
Happy studying guys!

finally, some content! this was a quick info graphic I drew up on Procreate to revise for my ochem test tomorrow. disclaimer: I used information from this source (https://www.masterorganicchemistry.com/2010/05/24/imines-and-enamines/) since my own notes are based off lectures I received at my university that I’m not really allowed to share without heavy modification.
general post disclaimer: I’m an undergraduate student studying biochemistry and genetics. Posts are made for the purposes of education, revision and aesthetics. Not all the content I produce can be taken as entirely accurate and I do not take responsibility for errors made as a result of using this resource. Always consult course textbooks and lectures to aid in your specific learning outcomes. Do not repost without the original caption citing any extra references I used to make this post or remove my watermark. Other posts can be found on my blog as-studypeach@tumblr.com. Any problems, feel free to get in touch via my messages.

If you are scrolling through Tumblr trying to distract yourself from something you don’t want to think about, or you’re looking for a sign. It is going to be okay. Just breathe. You are alive and you matter.


#OTD a year ago, Moderna’s RNA vaccine became the first #COVID19 vaccine to enter phase 1 trials. The latest #ChemVsCOVID graphic with the Royal Society of Chemistry takes a brief look at how prior research helped COVID vaccines reach this point quickly: https://ift.tt/3cE5xHR https://ift.tt/3rV4v0F
-
 gigipiet13 liked this · 3 years ago
gigipiet13 liked this · 3 years ago -
 wearestarsailors liked this · 4 years ago
wearestarsailors liked this · 4 years ago -
 drnmmt liked this · 4 years ago
drnmmt liked this · 4 years ago -
 amateurchemstudent reblogged this · 4 years ago
amateurchemstudent reblogged this · 4 years ago -
 amateurchemstudent liked this · 4 years ago
amateurchemstudent liked this · 4 years ago -
 monsterbaity liked this · 4 years ago
monsterbaity liked this · 4 years ago -
 dubiousspectrum reblogged this · 4 years ago
dubiousspectrum reblogged this · 4 years ago -
 itslunasapprentice liked this · 4 years ago
itslunasapprentice liked this · 4 years ago -
 skyfiresibs liked this · 4 years ago
skyfiresibs liked this · 4 years ago -
 alekin liked this · 4 years ago
alekin liked this · 4 years ago -
 lazyscience liked this · 4 years ago
lazyscience liked this · 4 years ago -
 dubiousspectrum liked this · 4 years ago
dubiousspectrum liked this · 4 years ago -
 sagansense reblogged this · 4 years ago
sagansense reblogged this · 4 years ago -
 inspirement liked this · 4 years ago
inspirement liked this · 4 years ago -
 cryptidcaper liked this · 4 years ago
cryptidcaper liked this · 4 years ago -
 sciencetylia reblogged this · 4 years ago
sciencetylia reblogged this · 4 years ago -
 cryptid-of-the-east liked this · 4 years ago
cryptid-of-the-east liked this · 4 years ago -
 zekaroundtheworld reblogged this · 4 years ago
zekaroundtheworld reblogged this · 4 years ago -
 mysticevening reblogged this · 4 years ago
mysticevening reblogged this · 4 years ago -
 mysticevening liked this · 4 years ago
mysticevening liked this · 4 years ago -
 simplyquetzo liked this · 4 years ago
simplyquetzo liked this · 4 years ago -
 crossedwithblue liked this · 4 years ago
crossedwithblue liked this · 4 years ago -
 orange-puzzline reblogged this · 4 years ago
orange-puzzline reblogged this · 4 years ago -
 heyfromthevoid liked this · 4 years ago
heyfromthevoid liked this · 4 years ago -
 big-fan-of-botw liked this · 4 years ago
big-fan-of-botw liked this · 4 years ago -
 shinxfranky reblogged this · 4 years ago
shinxfranky reblogged this · 4 years ago -
 0lex-x liked this · 4 years ago
0lex-x liked this · 4 years ago -
 badw0lfblue liked this · 4 years ago
badw0lfblue liked this · 4 years ago -
 japanerinihongo liked this · 4 years ago
japanerinihongo liked this · 4 years ago -
 wavewagger liked this · 4 years ago
wavewagger liked this · 4 years ago -
 mostly-adulting liked this · 4 years ago
mostly-adulting liked this · 4 years ago -
 kade0810 liked this · 4 years ago
kade0810 liked this · 4 years ago -
 dragoneggs52 liked this · 4 years ago
dragoneggs52 liked this · 4 years ago -
 msbiologist liked this · 4 years ago
msbiologist liked this · 4 years ago -
 uborkabor liked this · 4 years ago
uborkabor liked this · 4 years ago -
 autistically-lost-in-the-woods liked this · 4 years ago
autistically-lost-in-the-woods liked this · 4 years ago -
 lithewarrior liked this · 4 years ago
lithewarrior liked this · 4 years ago -
 swingineaglecopflower-blog liked this · 4 years ago
swingineaglecopflower-blog liked this · 4 years ago -
 thexfridax liked this · 4 years ago
thexfridax liked this · 4 years ago -
 sparklemiranda liked this · 4 years ago
sparklemiranda liked this · 4 years ago -
 rocks-and-ravens liked this · 4 years ago
rocks-and-ravens liked this · 4 years ago -
 qealo-blog liked this · 4 years ago
qealo-blog liked this · 4 years ago


