Rx Mnemonics
Rx Mnemonics
A nthrax = ACiD
Ci profloxacin
D oxycycline
Tr ichinellosis = TrAM
A lbendazole
M ebendazole
C utaneous Larva Migrans = CIA
I vermectin
A lbendazole
Le ptospirosis = LeAD
A moxicillin
D oxycycline
B rucellosis = BaRDS
Ba ctrim
R ifampicin
D oxycycline
S treptomycin
Ra t Bite Fever = RaPT
P enicillin
T etracycline
Ca t Scratch Disease = CaRAz
R ifampin
Az ithromycin
Ba besiosis = BAAz
A tovaquone
Az ithromycin
More Posts from T-b-a-blr-blog and Others


Me durning finals.

Some general resources:
Chemistry Glossary
Chemistry Exam Survival Guide
Toolbox – interactive graphing, tables, and calculators
Make virtual chemistry models
Interactive periodic table
Another site for making virtual chemistry models
Virtual labs – covers stoichiometry, thermochemistry, eq1uilibrium, acid base chemistry, solubility, oxidation/reduction and electrochemistry, analytical chemistry/lab techniques
Concept tests
Chemistry Science Fair Project Ideas
OChem Reaction Bank
Interactive chem simulations
Chemical calculations
The Chem Blog
Molecule of the day
Free chemistry drawing software
Laboratory Safety - Laboratory safety for the chemistry classroom
Periodic Table of Videos - Brady Haran
On this day in chemistry… - a history of chemistry
The faces of chemistry
Experimentation hub - explore and enjoy our experiments to increase engagement in scientific investigation, develop new skills and enhance your knowledge.
Understanding journals - including reading articles, referencing, and example articles.
Resources for specific topics:
Stochiometry – the mole, molarity and density, reaction stoichiometry and limiting reagents, empirical formula and mixtures, gravimetric analysis
Themochemistry – energy and enthalpy, entropy
Kinetics – phenomenological and mechanistic kinetics
Equilibrium – LeChatlier’s principle, progress of reaction, equilibrium calculations, common ion effect
Acid base chemistry – strong acid and bases, weak acids and bases, buffer solutions, acid/base titrations
Solubility – solubility product, solubility and PH, common ion effect
Oxidation/Reduction and Electrochemistry – standard reduction potentials, galvanic cells
Analytical chemistry/ Lab techniques – reaction stoichiometry and limiting reagents, acid/base titrations, redox titrations, gravimetric analysis, UC/Vis spectroscopy
Physical chemistry – quantum mechanics, spectroscopy
Properties of solutions – intermolecular forces, colligative properties
Textbooks:
Chemistry Virtual Textbooks, Stephen Lower
Organic Chemistry, Tim Soderberg
Organic Chemistry I, George Mhehe
Environmental Chemistry, Dejene Tessema
Virtual Organic Chemistry
Industrial Chemistry, Helen Njenga
Inorganic Chemistry, Chrispin Kowenje
Physical Chemistry I, Onesmus Munyaki
General Chemistry, Principles, Patterns and Applications
Chemistry Books - a variety of chemistry textbooks
Chemistry Tutorials/Guides:
Atoms, Molecules, and Ions
Chemical reactions and stoichiometry
Electronic structure of atoms
Periodic table
Chemical bonds
Gases and kinetic molecular theory
State of matter and intermolecular forces
Chemical equilibrium
Acids and bases
Acid base equilibria and solubility equilibria
Thermodynamics
Redox reactions and electrochemistry
Kinetics
Nuclear chemistry
Organic Chemistry Tutorials/Guidelines:
Structure and bonding
Dot structures
Hybridization
Bond-line structures
Electronegativity
Resonance and acid base chemistry
Counting electrons
Resonance structures
Organic acid-base chemistry
Alkanes, cycloalkanes and functional groups
Naming alkanes
Naming alkanes, cycloalkanes, and bicyclic compounds
Conformations of alkanes
Conformations of cycloalkanes
Functional groups
Stereochemistry
Chirality
Enantiomers
Stereoisomeric relationships
Subsituation and elimination reactions
Free radical reaction
Sn1 vs Sn2
Nucleophilicity and basicity
Elimination reactions
Sn1/Sn2/E1/E2
Sn1 and Sn2
Alkenes and alkynes
Naming alkenes
Alkene reactions
Alkene nomenclature
Alkene reactions
Naming and preparing alkynes
Alkyne reactions
Alcohols, ethers, epoxides, sulphides
Alcohol nomenclature and properties
Synthesis of alcohols
Reactions of alcohols
Nomenclature and properties of ethers
Synthesis and cleavage of ethers
Nomenclature and preparation of epoxides
Conjugation, Diels-Alder, and MO theory
Addition reactions of conjugated dienes
Diels-Alder reaction
Molecular orbital theory
Aromatic compounds
Naming benzene derivatives
Reactions of benzene
Aromatic stability
Electrophilic aromatic substitution
Directing effects
Other reactions and synthesis
Aldehydes and ketones
Introduction to aldehydes and ketones
Reactions of aldehydes and ketones
Carboxylic acids and derivatives
Naming carboxylic acids
Formation of carboxylic acid derivatives
Nomenclature and reactions of carboxylic acids
Nomenclature and reactions of carboxylic acid derivatives
Alpha carbon chemistry
Formation of enolate anions
Aldol condensations
Amines
Naming amines
Spectroscopy
Infrared Spectroscopy
UV/Vis Spectroscopy
proton NMR
Careers:
A future in Chemistry
What can I do with my chemistry degree?
Chemistry Careers - American Chemical Society
What to do with a degree in chemistry - The Guardian
Antibodies (Human)

The ‘foot’ (bottom) of the antibody is known as the Fc fragment - binds to cells, binds to complement = effector function (kills or removes antigen)
The top (antigen binding) is the Fab fragment
Chains are held together with disulphide binds
Associated molecules allow intracellular signalling
Normally 3X constant heavy chain domains per chain and a hinge region (except μ and ε which have 4 and no hinge region)
Classes of Immunoglobulins
The five primary classes of immunoglobulins are IgG, IgM, IgA, IgD and IgE, distinguished by the type of heavy chain found in the molecule.
IgG - gamma-chains
IgMs - mu-chains
IgAs - alpha-chains
IgEs - epsilon-chains
IgDs - delta-chains.
Differences in heavy chain polypeptides allow different types of immune responses. The differences are found primarily in the Fc fragment. There are only two main types of light chains: kappa (κ) and lambda (λ), and any antibody can have any combination of these 2 (variation).
IgG
monomer
Gamma chains
70-85% of Ig in human serum.
secondary immune response
only class that can cross the placenta - protection of the newborn during first 6 months of life
principle antibody used in immunological research and clinical diagnostics
21 day half life
Hinge region (allows it to make Y and T shapes - increasing chance of being able to bind to more than one site)
Fc strongly binds to Fcγ receptor on phagocyte - opsono-phagocytosis
Activates complement pathway

IgM
Serum = pentamer
Primary immune responses - first Ig to be synthesised
complement fixing
10% of serum Ig
also expressed on the plasma membrane of B lymphocytes as a monomer - B cell antigen receptor
H chains each contain an additional hydrophobic domain for anchoring in the membrane
Monomers are bound together by disulfide bonds and a joining (J) chain.
Each of the five monomers = two light chains (either kappa or lambda) and two mu heavy chains.
heavy chain = one variable and four constant regions (no hinge region)
can cause cell agglutination as a result of recognition of epitopes on invading microorganisms. This antibody-antigen immune complex is then destroyed by complement fixation or receptor mediated endocytosis by macrophages.
In humans there are four subclasses of IgG: IgG1, IgG2, IgG3 and IgG4. IgG1 and IgG3 activate complement.

IgD
B cell receptor
<1% of blood serum Ig
has tail pieces that anchor it across B cell membrane
forms an antigen specific receptor on mature B cells - consequently has no known effector function (don’t kill antigens, purely a receptor) (IgM as a monomer can also do this)

IgE
Extra rigid central domain
has the most carbohydrates
IgE primarily defends against parasitic invasion and is responsible for allergic reactions.
basophils and tissue mast cells express very high affinity Fc receptors for IgE - mast cells then release histamine
so high that almost all IgE is bound
sensitizes (activates) mucosal cells and tissues
protects against helminth parasites
IgE’s main purpose is to protect against parasites but due to improved sanitation these are no longer a prevalent issue across most of the world. Consequently it is thought that they become over activated and over sensitive while looking for parasites and start reacting to eg pollen and causing allergies.

IgA
Exists in serum in both monomeric (IgA1) and dimeric (IgA2) forms (dimeric when 2 Fcs bind via secretory complex)
15% of the total serum Ig.
4-7 day half life
Secretory IgA2 (dimer) = primary defense against some local infections
Secreted as a dimer in mucous (e.g., saliva, tears)
prevents passage of foreign substances into the circulatory system


Isotype: class of antibody (IgD, IgM etc)
Allotype: person specific alleles
Idiotype: (hyper) variable region - antibody specificity
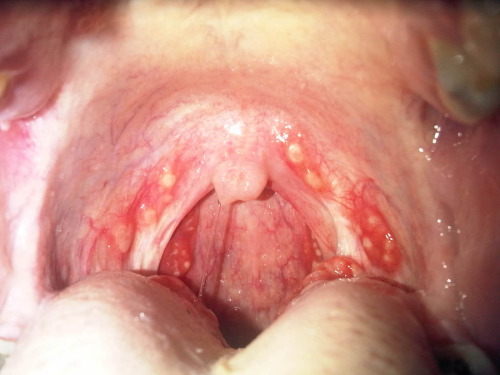
Strep throat.




12.3.17
When your teacher said they’re going to post a study guide but they don’t qq
Music mood: Rubber Human - Mili


I made a lymph drainage sticker for the immuno section of first aid
You can get the Sticker here: https://www.redbubble.com/people/histrionicole/works/29980305-lymph-node-drainage?asc=u&p=sticker
Biochem enzymes MNEMONIC
“ABC” enzymes


.
“TLC For Nancy” (TLCFN) Coenzymes & Cofactors


.
Thiamine PyroPhosphate -TPP- requiring enzymes (B1)


.
Pirydoxine (B6) coenzyme


.
Cyanocobalamine (B12) coenzyme


.
Succynil CoA source: “VOMIT”



Stuff in my Office
From 1930. This well-dressed young scientist is experimenting with The Air. Exactly what he is doing is a mystery.
July 25 …. I finally get it! He’s holding an eye dropper with a bulb on the end. I thought it was a pair of chopsticks! He’s picking up pieces of paper using the vacuum from squeezing the bulb! Still very formal, though …

-
 nicolenoav liked this · 1 year ago
nicolenoav liked this · 1 year ago -
 gprswa liked this · 1 year ago
gprswa liked this · 1 year ago -
 ninja-trap liked this · 1 year ago
ninja-trap liked this · 1 year ago -
 scenteddeerpizza liked this · 2 years ago
scenteddeerpizza liked this · 2 years ago -
 the24hrtherapist liked this · 3 years ago
the24hrtherapist liked this · 3 years ago -
 kchaitali23 reblogged this · 4 years ago
kchaitali23 reblogged this · 4 years ago -
 live-love-lightly liked this · 4 years ago
live-love-lightly liked this · 4 years ago -
 paureus reblogged this · 5 years ago
paureus reblogged this · 5 years ago -
 devinfreetime liked this · 5 years ago
devinfreetime liked this · 5 years ago -
 angelaellar reblogged this · 5 years ago
angelaellar reblogged this · 5 years ago -
 katwalsubash-blog liked this · 6 years ago
katwalsubash-blog liked this · 6 years ago -
 bajiraokaushal-blog liked this · 6 years ago
bajiraokaushal-blog liked this · 6 years ago -
 leblogdepetites liked this · 6 years ago
leblogdepetites liked this · 6 years ago -
 t-b-a-blr-blog reblogged this · 6 years ago
t-b-a-blr-blog reblogged this · 6 years ago -
 t-b-a-blr-blog liked this · 6 years ago
t-b-a-blr-blog liked this · 6 years ago -
 zandersonkcom liked this · 7 years ago
zandersonkcom liked this · 7 years ago -
 pharmacyhun reblogged this · 7 years ago
pharmacyhun reblogged this · 7 years ago -
 trypanosomacrazy7-blog liked this · 7 years ago
trypanosomacrazy7-blog liked this · 7 years ago -
 ferlistudyblr reblogged this · 8 years ago
ferlistudyblr reblogged this · 8 years ago -
 hanejar-blog liked this · 8 years ago
hanejar-blog liked this · 8 years ago -
 paolocabeza-blog liked this · 8 years ago
paolocabeza-blog liked this · 8 years ago -
 bradykinetics reblogged this · 8 years ago
bradykinetics reblogged this · 8 years ago -
 bradykinetics liked this · 8 years ago
bradykinetics liked this · 8 years ago -
 drumarafridi22 liked this · 9 years ago
drumarafridi22 liked this · 9 years ago -
 bqhhj reblogged this · 9 years ago
bqhhj reblogged this · 9 years ago -
 bqhhj liked this · 9 years ago
bqhhj liked this · 9 years ago -
 adventuresofamedhead liked this · 11 years ago
adventuresofamedhead liked this · 11 years ago -
 diaahamouda liked this · 11 years ago
diaahamouda liked this · 11 years ago -
 usmle1mikmonics reblogged this · 11 years ago
usmle1mikmonics reblogged this · 11 years ago -
 gonnabeanmd reblogged this · 11 years ago
gonnabeanmd reblogged this · 11 years ago -
 mkvictor19 liked this · 11 years ago
mkvictor19 liked this · 11 years ago -
 flomelyhs reblogged this · 11 years ago
flomelyhs reblogged this · 11 years ago -
 valkyrie4eva-blog reblogged this · 11 years ago
valkyrie4eva-blog reblogged this · 11 years ago -
 schwann86-blog reblogged this · 11 years ago
schwann86-blog reblogged this · 11 years ago -
 dr-farid reblogged this · 11 years ago
dr-farid reblogged this · 11 years ago -
 dr-farid liked this · 11 years ago
dr-farid liked this · 11 years ago -
 caseofthegiggles reblogged this · 11 years ago
caseofthegiggles reblogged this · 11 years ago -
 akshay03 liked this · 11 years ago
akshay03 liked this · 11 years ago -
 inter-anastomoses liked this · 11 years ago
inter-anastomoses liked this · 11 years ago -
 heyvivian liked this · 11 years ago
heyvivian liked this · 11 years ago