Tularemia
Tularemia
An infection common in wild rodents that is passed to humans through contact with infected animal tissues or by ticks, biting flies, and mosquitoes.
Also known as rabbit fever and deer fly fever, amongst others.

More Posts from T-b-a-blr-blog and Others

volutin granules are an intracytoplasmic storage form of complexed inorganic polyphosphate, the production of which is used as one of the identifying criteria when attempting to isolate Corynebacterium diphtheriae on Löffler’s medium….look like chines letters…as given below

Different anatomy notes form this semester Supplies used (not all at once, I mix and match): Faber-Castell Coloured Pencils (48 Pack) - https://amzn.to/2Kd1mUy Staedtler Triplus Fineliners - http://amzn.to/2pghonI Stabilo Point 88 Fineliner - https://amzn.to/2qU8fC9 Sharpie Pens - https://amzn.to/2HTRmP2 Uni Pin 0.1 Fineliner - https://amzn.to/2HmXp1z Crayola Supertips - https://amzn.to/2HVW1jr Bic Ballpoint Pen - https://amzn.to/2HmCjk0 Stabilo Swing Cool Highlighters - https://amzn.to/2HKxPTu




• Microbiology cheat sheets for the comprehensive final. We were allowed two pages, front and back. I ended up getting a 95%! •
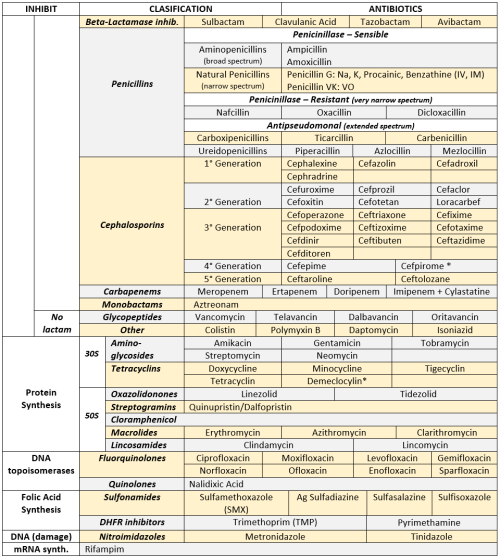
ANTIBIOTICS CHEAT SHEET :)
Also, REMEMBER!!!!
* Sulfonamides compete for albumin with:
Bilirrubin: given in 2°,3°T, high risk or indirect hyperBb and kernicterus in premies
Warfarin: increases toxicity: bleeding
* Beta-lactamase (penicinillase) Suceptible:
Natural Penicillins (G, V, F, K)
Aminopenicillins (Amoxicillin, Ampicillin)
Antipseudomonal Penicillins (Ticarcillin, Piperacillin)
* Beta-lactamase (penicinillase) Resistant:
Oxacillin, Nafcillin, Dicloxacillin
3°G, 4°G Cephalosporins
Carbapenems
Monobactams
Beta-lactamase inhibitors
* Penicillins enhanced with:
Clavulanic acid & Sulbactam (both are suicide inhibitors, they inhibit beta-lactamase)
Aminoglycosides (against enterococcus and psedomonas)
* Aminoglycosides enhanced with Aztreonam
* Penicillins: renal clearance EXCEPT Oxacillin & Nafcillin (bile)
* Cephalosporines: renal clearance EXCEPT Cefoperazone & Cefrtriaxone (bile)
* Both inhibited by Probenecid during tubular secretion.
* 2°G Cephalosporines: none cross BBB except Cefuroxime
* 3°G Cephalosporines: all cross BBB except Cefoperazone bc is highly highly lipid soluble, so is protein bound in plasma, therefore it doesn’t cross BBB.
* Cephalosporines are "LAME“ bc they do not cover this organisms
L isteria monocytogenes
A typicals (Mycoplasma, Chlamydia)
M RSA (except Ceftaroline, 5°G)
E nterococci

* Disulfiram-like effect: Cefotetan & Cefoperazone (mnemonic)
* Cefoperanzone: all the exceptions!!!
All 3°G cephalosporins cross the BBB except Cefoperazone.
All cephalosporins are renal cleared, except Cefoperazone.
Disulfiram-like effect
* Against Pseudomonas:
3°G Cef taz idime (taz taz taz taz)
4°G Cefepime, Cefpirome (not available in the USA)
Antipseudomonal penicillins
Aminoglycosides (synergy with beta-lactams)
Aztreonam (pseudomonal sepsis)
* Covers MRSA: Ceftaroline (rhymes w/ Caroline, Caroline the 5°G Ceph), Vancomycin, Daptomycin, Linezolid, Tigecycline.
* Covers VRSA: Linezolid, Dalfopristin/Quinupristin
* Aminoglycosides: decrease release of ACh in synapse and act as a Neuromuscular blocker, this is why it enhances effects of muscle relaxants.
* DEMECLOCYCLINE: tetracycline that’s not used as an AB, it is used as tx of SIADH to cause Nephrogenic Diabetes Insipidus (inhibits the V2 receptor in collecting ducts)
* Phototoxicity: Q ue S T ion?
Q uinolones
Sulfonamides
T etracyclines

* p450 inhibitors: Cloramphenicol, Macrolides (except Azithromycin), Sulfonamides
* Macrolides SE: Motilin stimulation, QT prolongation, reversible deafness, eosinophilia, cholestatic hepatitis
* Bactericidal: beta-lactams (penicillins, cephalosporins, monobactams, carbapenems), aminoglycosides, fluorquinolones, metronidazole.
* Baceriostatic: tetracyclins, streptogramins, chloramphenicol, lincosamides, oxazolidonones, macrolides, sulfonamides, DHFR inhibitors.
* Pseudomembranous colitis: Ampicillin, Amoxicillin, Clindamycin, Lincomycin.
* QT prolongation: macrolides, sometimes fluoroquinolones
Archaea
Archaeans are single-celled and join bacteria to make up the Prokaryotes. The Archaea classification is a very recent discovery, due to the similarities in appearance and behaviour to bacteria they weren’t separated until the late 1970′s. They mostly live in extreme environments and can be sub grouped:
Methanogens - produce methane gas as a waste product of their “digestion,” or process of making energy.
Halophiles - live in salty environments.
Thermophiles - live at extremely hot temperatures.
Psychrophiles — those that live at unusually cold temperatures.
Like bacteria, archaea lack a true nucleus. Both bacteria and archaea usually have one DNA molecule suspended in the cell’s cytoplasm contained within a cell membrane. Most, but not all, have a tough, rigid outer cell wall.

use a variety of substances for energy, including hydrogen gas, carbon dioxide and sulfur.
many archaea thrive in conditions mimicking those found more than 3.5 billion years ago. [eg oceans that regularly reached boiling point — an extreme condition not unlike the hydrothermal vents and sulfuric waters where archaea are found today]

Some general resources:
Chemistry Glossary
Chemistry Exam Survival Guide
Toolbox – interactive graphing, tables, and calculators
Make virtual chemistry models
Interactive periodic table
Another site for making virtual chemistry models
Virtual labs – covers stoichiometry, thermochemistry, eq1uilibrium, acid base chemistry, solubility, oxidation/reduction and electrochemistry, analytical chemistry/lab techniques
Concept tests
Chemistry Science Fair Project Ideas
OChem Reaction Bank
Interactive chem simulations
Chemical calculations
The Chem Blog
Molecule of the day
Free chemistry drawing software
Laboratory Safety - Laboratory safety for the chemistry classroom
Periodic Table of Videos - Brady Haran
On this day in chemistry… - a history of chemistry
The faces of chemistry
Experimentation hub - explore and enjoy our experiments to increase engagement in scientific investigation, develop new skills and enhance your knowledge.
Understanding journals - including reading articles, referencing, and example articles.
Resources for specific topics:
Stochiometry – the mole, molarity and density, reaction stoichiometry and limiting reagents, empirical formula and mixtures, gravimetric analysis
Themochemistry – energy and enthalpy, entropy
Kinetics – phenomenological and mechanistic kinetics
Equilibrium – LeChatlier’s principle, progress of reaction, equilibrium calculations, common ion effect
Acid base chemistry – strong acid and bases, weak acids and bases, buffer solutions, acid/base titrations
Solubility – solubility product, solubility and PH, common ion effect
Oxidation/Reduction and Electrochemistry – standard reduction potentials, galvanic cells
Analytical chemistry/ Lab techniques – reaction stoichiometry and limiting reagents, acid/base titrations, redox titrations, gravimetric analysis, UC/Vis spectroscopy
Physical chemistry – quantum mechanics, spectroscopy
Properties of solutions – intermolecular forces, colligative properties
Textbooks:
Chemistry Virtual Textbooks, Stephen Lower
Organic Chemistry, Tim Soderberg
Organic Chemistry I, George Mhehe
Environmental Chemistry, Dejene Tessema
Virtual Organic Chemistry
Industrial Chemistry, Helen Njenga
Inorganic Chemistry, Chrispin Kowenje
Physical Chemistry I, Onesmus Munyaki
General Chemistry, Principles, Patterns and Applications
Chemistry Books - a variety of chemistry textbooks
Chemistry Tutorials/Guides:
Atoms, Molecules, and Ions
Chemical reactions and stoichiometry
Electronic structure of atoms
Periodic table
Chemical bonds
Gases and kinetic molecular theory
State of matter and intermolecular forces
Chemical equilibrium
Acids and bases
Acid base equilibria and solubility equilibria
Thermodynamics
Redox reactions and electrochemistry
Kinetics
Nuclear chemistry
Organic Chemistry Tutorials/Guidelines:
Structure and bonding
Dot structures
Hybridization
Bond-line structures
Electronegativity
Resonance and acid base chemistry
Counting electrons
Resonance structures
Organic acid-base chemistry
Alkanes, cycloalkanes and functional groups
Naming alkanes
Naming alkanes, cycloalkanes, and bicyclic compounds
Conformations of alkanes
Conformations of cycloalkanes
Functional groups
Stereochemistry
Chirality
Enantiomers
Stereoisomeric relationships
Subsituation and elimination reactions
Free radical reaction
Sn1 vs Sn2
Nucleophilicity and basicity
Elimination reactions
Sn1/Sn2/E1/E2
Sn1 and Sn2
Alkenes and alkynes
Naming alkenes
Alkene reactions
Alkene nomenclature
Alkene reactions
Naming and preparing alkynes
Alkyne reactions
Alcohols, ethers, epoxides, sulphides
Alcohol nomenclature and properties
Synthesis of alcohols
Reactions of alcohols
Nomenclature and properties of ethers
Synthesis and cleavage of ethers
Nomenclature and preparation of epoxides
Conjugation, Diels-Alder, and MO theory
Addition reactions of conjugated dienes
Diels-Alder reaction
Molecular orbital theory
Aromatic compounds
Naming benzene derivatives
Reactions of benzene
Aromatic stability
Electrophilic aromatic substitution
Directing effects
Other reactions and synthesis
Aldehydes and ketones
Introduction to aldehydes and ketones
Reactions of aldehydes and ketones
Carboxylic acids and derivatives
Naming carboxylic acids
Formation of carboxylic acid derivatives
Nomenclature and reactions of carboxylic acids
Nomenclature and reactions of carboxylic acid derivatives
Alpha carbon chemistry
Formation of enolate anions
Aldol condensations
Amines
Naming amines
Spectroscopy
Infrared Spectroscopy
UV/Vis Spectroscopy
proton NMR
Careers:
A future in Chemistry
What can I do with my chemistry degree?
Chemistry Careers - American Chemical Society
What to do with a degree in chemistry - The Guardian



MORE MIXED MNEMONICS
Penicillin

Penicillin is a widely used antibiotic prescribed to treat staphylococci and streptococci bacterial infections.
beta-lactam family
Gram-positive bacteria = thick cell walls containing high levels of peptidoglycan
gram-negative bacteria = thinner cell walls with low levels of peptidoglycan and surrounded by a lipopolysaccharide (LPS) layer that prevents antibiotic entry
penicillin is most effective against gram-positive bacteria where DD-transpeptidase activity is highest.
Examples of penicillins include:
amoxicillin
ampicillin
bacampicillin
oxacillin
penicillin
Mechanism(s)
Penicillin inhibits the bacterial enzyme transpeptidase, responsible for catalysing the final peptidoglycan crosslinking stage of bacterial cell wall synthesis.
Cells wall is weakened and cells swell as water enters and then burst (lysis)
Becomes permanently covalently bonded to the enzymes’s active site (irreversible)


Alternative theory: penicillin mimics D-Ala D-Ala

Or may act as an umbrella inhibitor

Resistance
production of beta-lactamase - destroys the beta-lactam ring of penicillin and makes it ineffective (eg Staphylococcus aureus - most are now resistant)
In response, synthetic penicillin that is resistant to beta-lactamase is in use including egdicloxacillin, oxacillin, nafcillin, and methicillin.
Some is resistant to methicillin - methicillin-resistant Staphylococcus aureus (MRSA).
Demonstrating blanket resistance to all beta-lactam antibiotics -extremely serious health risk.
-
 devinfreetime liked this · 5 years ago
devinfreetime liked this · 5 years ago -
 angelaellar reblogged this · 5 years ago
angelaellar reblogged this · 5 years ago -
 t-b-a-blr-blog reblogged this · 6 years ago
t-b-a-blr-blog reblogged this · 6 years ago -
 t-b-a-blr-blog liked this · 6 years ago
t-b-a-blr-blog liked this · 6 years ago -
 vibgyorbatman liked this · 7 years ago
vibgyorbatman liked this · 7 years ago -
 paolocabeza-blog reblogged this · 8 years ago
paolocabeza-blog reblogged this · 8 years ago -
 paolocabeza-blog liked this · 8 years ago
paolocabeza-blog liked this · 8 years ago -
 yazz-r reblogged this · 10 years ago
yazz-r reblogged this · 10 years ago -
 mynotes4usmle liked this · 11 years ago
mynotes4usmle liked this · 11 years ago -
 bjnursing reblogged this · 11 years ago
bjnursing reblogged this · 11 years ago -
 shiloedenrose liked this · 11 years ago
shiloedenrose liked this · 11 years ago -
 imtiazdanny liked this · 11 years ago
imtiazdanny liked this · 11 years ago -
 ob-nurse--at-your-cervix liked this · 11 years ago
ob-nurse--at-your-cervix liked this · 11 years ago -
 ob-nurse--at-your-cervix reblogged this · 11 years ago
ob-nurse--at-your-cervix reblogged this · 11 years ago -
 idyllicrealm liked this · 11 years ago
idyllicrealm liked this · 11 years ago -
 nurse-on-duty reblogged this · 11 years ago
nurse-on-duty reblogged this · 11 years ago -
 archiveofbiognosis liked this · 11 years ago
archiveofbiognosis liked this · 11 years ago -
 nakimedicalblog reblogged this · 11 years ago
nakimedicalblog reblogged this · 11 years ago -
 yasasiihitogomi reblogged this · 12 years ago
yasasiihitogomi reblogged this · 12 years ago -
 yasasiihitogomi liked this · 12 years ago
yasasiihitogomi liked this · 12 years ago -
 pedicabo-ego-vos-et-irrumabo reblogged this · 12 years ago
pedicabo-ego-vos-et-irrumabo reblogged this · 12 years ago -
 futurespinsterdoctor liked this · 12 years ago
futurespinsterdoctor liked this · 12 years ago -
 7-taher reblogged this · 12 years ago
7-taher reblogged this · 12 years ago -
 medicineisnotmerchandise liked this · 12 years ago
medicineisnotmerchandise liked this · 12 years ago -
 mynotes4usmle reblogged this · 12 years ago
mynotes4usmle reblogged this · 12 years ago -
 medicalthings-blog reblogged this · 12 years ago
medicalthings-blog reblogged this · 12 years ago
