Dive Deep into Creativity: Discover, Share, Inspire
Carbon - Blog Posts
Alkanes: Saturated Hydrocarbons
So you want to be an organic chemist? Well, learning about hydrocarbons such as alkanes is a good place to start…
Alkanes are a homologous series of hydrocarbons, meaning that each of the series differs by -CH2 and that the compounds contain carbon and hydrogen atoms only. Carbon atoms in alkanes have four bonds which is the maximum a carbon atom can have - this is why the molecule is described to be saturated. Saturated hydrocarbons have only single bonds between the carbon atoms.
The general formula of an alkane is CnH2n+2 where n is the number of carbons. For example, if n = 3, the hydrocarbon formula would be C3H8 or propane. Naming alkanes comes from the number of carbons in the chain structure.
Here are the first three alkanes. Each one differs by -CH2.

Shorter chain alkanes are gases at room temperature, medium ones are liquids and the longer chain alkanes are waxy solids.
Alkanes have these physical properties:
1. They are non-polar due to the tiny difference in electronegativity between the carbon and hydrogen atoms.
2. Only Van der Waals intermolecular forces exist between alkane molecules. The strength of these increase as relative molecular mass increases therefore so does the melting/boiling point.
3. Branched chain alkanes have lower melting and boiling points than straight chain isomers with the same number of carbons. Since atoms are further apart due to a smaller surface area in contact with each other, the strength of the VDWs is decreased.
4. Alkanes are insoluble in water but can dissolve in non-polar liquids like hexane and cyclopentane. Mixtures are separated by fractional distillation or a separating funnel.
The fractional distillation of crude oil, cracking and the combustion equations of the alkanes will be in the next post.
SUMMARY
Alkanes are a homologous series of hydrocarbons. Carbon atoms in alkanes have four bonds which is the maximum a carbon atom can have - this is why the molecule is described to be saturated. Saturated hydrocarbons have only single bonds between the carbon atoms.
The general formula of an alkane is CnH2n+2 where n is the number of carbons.
Shorter chain alkanes are gases at room temperature, medium ones are liquids and the longer chain alkanes are waxy solids.
They are non-polar.
Only Van der Waals intermolecular forces exist between alkane molecules. The strength of these increase as relative molecular mass increases therefore so does the melting/boiling point.
Branched chain alkanes have lower melting and boiling points than straight chain isomers with the same number of carbons.
Alkanes are insoluble in water but can dissolve in non-polar liquids like hexane. Mixtures are separated by fractional distillation or a separating funnel.
Haloalkanes and Their Angelic Reactions: Part One
Haloalkanes are more commonly referred to as halogenoalkanes. Obviously you’ve already read my post on halogenoalkanes and their properties so there’s no surprise that you’re itching to read what I’ve got to say about these beauties and their reactions! Should we delve in?
There are a few different kinds of reactions you must learn for the A Level exam that involve halogenoalkanes.
The first is the synthesis of chloroalkanes via the photochemical chlorination of the alkanes. I know it looks scary, but don’t worry, it is simpler than it sounds. It essentially means “forming chloroalkanes through chlorinating an alkane in the presence of sunlight”.
Chlorine will react with methane when UV light is present and will form several kinds of chloroalkanes and fumes of hydrogen chloride gas. Chloromethane was once commonly used as a refridgerant. Depending on how many chlorine molecules there are, there will be different compounds formed:
methane + chlorine -> chloromethane + hydrogen chloride
CH4 + Cl2 -> CH3Cl + HCl
or
methane + chlorine -> trichloromethane + hydrogen chloride
CH4 + 3Cl2 -> CHCl3 + 3HCl
When undergone in real life, mixtures of halogenoalkanes are produced with some long chain alkanes which can be separated out with fractional distillation.
To understand what happens in an overall chemical reaction, chemists use mechanisms. These basically show the step-by-step process that is usually shown by a simple symbol equation that summarises everything.
The chlorination of methane is something you must learn the mechanism for. It’s pretty easy but involves a lot of steps and must be revised periodically to remember them.
The actual reaction is a substitution reaction because one atom or group is replaced by another. Since the chlorine involved is a free radical, it can also be called a free-radical substitution reaction.
1. Initiation
UV light is essential for the first step in the mechanism. This breaks the Cl-Cl covalent bond so that each chlorine leaves with one electron from the shared pair. Chlorine free radicals, with one unpaired electron in the outer shell, are formed. Free radicals are only formed if a bond splits evenly - each atom getting one of the two electrons. The name given to this is homolytic fission.

2. Propagation
This has two sub-steps
(a) Chlorine free radicals (highly reactive) react with methane to form hydrogen chloride and leave a methyl free radical.
Cl• + CH4 -> HCl + •CH3
(b) This free radical then reacts with another chlorine to form chloromethane and another chlorine free radical. Producing free radicals is a chain reaction which is why it is such a problem in ozone depletion - a little amount can cause a lot of destruction.
•CH3 + Cl2 -> CH3Cl + •Cl
3. Termination
This step stops the chain reaction. It only happens when two free radicals collide to form a molecule in several ways:
Cl• + Cl• -> Cl2
UV light would just break down the chlorine molecule again, so although this is technically a termination reaction it is not the most efficient.
Cl• + •CH3 -> CH3Cl
Forming one molecule of methane uses one chlorine and one methyl free radical.
•CH3 + •CH3 -> C2H6
Ethane can be formed from two methyl free radicals - this is why there are longer chain alkanes in the mixture.
This whole process is how organic halogenoalkanes are the product of photochemical reactions of halogens with alkanes in UV light - made via free radical substitution mechanisms in chain reaction.
Another reaction you need to know is a nucleophilic substitution reactions. A nucleophile is an electron pair donor or proton acceptor - the name comes from Greek origins (”loves nucleus”) - such as hydroxide ions, cyanide ions or ammonia molecules. Hydroxide and cyanide ions are negative but ammonia is neutral.

Halogenoalkanes have a polar bond because of the difference between the highly electronegative halogen and the carbon atom. The 𝛿+ carbon can go under nucleophilic attack. The mechanism for negatively charged nucleophiles these in general is:

Nu represents the nucleophile. This example is with a bromoalkane. Make sure to include curly arrows that begin at a lone pair or the centre of a bond and end at an atom or centre of bond, and delta (slight) charges.
Lets look at a more specific example:
One nucleophile that can be used is a hydroxide ion, found in either water or sodium hydroxide. In this case, you need to know about aqueous sodium hydroxide or potassium hydroxide and a halogenoalkane. This takes place at room temperature but is slow so is often refluxed (continuously boiled and condensed back into the reaction flask). Reflux apparatus is shown below:

The halogenoalkane is dissolved into ethanol since it is insoluable in water and this solution along with the aqueous hydroxide can mix. The product produced is an alcohol, which is organic.
The general reaction is:
R-CH2X + NaOH -> CH3CH2OH + NaX
Where X represents a halogen.
You must learn the mechanism for this reaction. The lone pair on the hydroxide attacks the carbon atom attached to the halogen and this causes both carbon electrons to move to the halogen which becomes a halide ion.

The reaction of a hydroxide ion can also be classed as a hydrolysis reaction as it breaks down chemical bonds with water or hydroxide ions. The speed of reaction depends on the strength of the bond - a stronger carbon-halogen bond, a slower reaction.

C-I is the most reactive (reactivity increases down group 7) and C-F is therefore the least reactive and strongest.
Part two of this post will cover nucleophilic substitution of cyanide ions and ammonia molecules, as well as elimination reactions.
SUMMARY
You need to know about the synthesis of chloroalkanes via the photochemical chlorination of the alkanes. - “forming chloroalkanes through chlorinating an alkane in the presence of sunlight”.
Chlorine will react with methane when UV light is present and will form several kinds of chloroalkanes and fumes of hydrogen chloride gas. Depending on how many chlorine molecules there are, there will be different compounds formed.
When undergone in real life, mixtures of halogenoalkanes are produced with some long chain alkanes which can be separated out with fractional distillation.
To understand what happens in an overall chemical reaction, chemists use mechanisms. These basically show the step-by-step process.
The chlorination of methane is something you must learn the mechanism for. The actual reaction is a substitution reaction because one atom or group is replaced by another.
The first step is initiation - UV light is essential for the first step in the mechanism. This breaks the Cl-Cl covalent bond so that each chlorine leaves with one electron from the shared pair. Chlorine free radicals, with one unpaired electron in the outer shell, are formed. Free radicals are only formed if a bond splits evenly - each atom getting one of the two electrons.
Step two is propagation: (a) Chlorine free radicals (highly reactive) react with methane to form hydrogen chloride and leave a methyl free radical (b) this free radical then reacts with another chlorine to form chloromethane and another chlorine free radical. Producing free radicals is a chain reaction which is why it is such a problem in ozone depletion - a little amount can cause a lot of destruction.
To stop the chain reaction, the final step is termination. It only happens when two free radicals collide to form a molecule in several ways: two chlorine free radicals forming a chlorine molecule, two methyl FRs forming ethane or a chlorine FR and a methyl FR forming chloromethane.
Ethane contributes to the longer chain alkanes in the mixture.
Another reaction you need to know is a nucleophilic substitution reactions. A nucleophile is an electron pair donor or proton acceptor, such as hydroxide ions, cyanide ions or ammonia molecules. Hydroxide and cyanide ions are negative but ammonia is neutral.
Halogenoalkanes have a polar bond because of the difference between the highly electronegative halogen and the carbon atom. The 𝛿+ carbon can go under nucleophilic attack.
Nu represents the nucleophile. Make sure to include curly arrows that begin at a lone pair or the centre of a bond and end at an atom or centre of bond, and delta (slight) charges.
One nucleophile that can be used is a hydroxide ion, found in either water or sodium hydroxide. In this case, you need to know about aqueous sodium hydroxide or potassium hydroxide and a halogenoalkane. This takes place at room temperature but is slow so is often refluxed (continuously boiled and condensed back into the reaction flask). The halogenoalkane is dissolved into ethanol since it is insoluable in water and this solution along with the aqueous hydroxide can mix. The product produced is an alcohol, which is organic.
The general reaction is :R-CH2X + NaOH -> CH3CH2OH + NaX where X represents a halogen
The lone pair on the hydroxide attacks the carbon atom attached to the halogen and this causes both carbon electrons to move to the halogen which becomes a halide ion.
The reaction of a hydroxide ion can also be classed as a hydrolysis reaction as it breaks down chemical bonds with water or hydroxide ions.
The speed of reaction depends on the strength of the bond - a stronger carbon-halogen bond, a slower reaction. C-I is the most reactive (reactivity increases down group 7) and C-F is therefore the least reactive and strongest.
Covalent Bonds: Sharing Is Caring!
Welcome to my second out of three posts on bonding - ionic, covalent and metallic. This post also covers the coordinate/ dative bond which I can’t remember if I’ve covered before. Only one more of this series left! Find the others here.
Covalent bonding involves one or more shared pairs of electrons between two atoms. These can be found in simple molecular elements and compounds like CO2 , macromolecular structures like diamond and molecular ions such as ammonium. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms – double and triple bonds represented with two and three lines respectively.
Dot and cross diagrams show the arrangement of electrons in covalent bonds. They use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.

The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds. For example, if an atom wants to make three covalent bonds but has a full 3s2 shell and a 3p1 shell, it can promote one of its 3s2 electrons so that an electron from the other atoms can fill the 3s shell and pair with the new 3p2 shell.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.

A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.

This is an example of a coordinate (sometimes called dative) bond between ammonia and a H+ ion which has an empty orbital. The lone pair on the ammonia overlaps with this H+ ion and donates its electrons. Both electrons come from the ammonia’s lone pair so it is a coordinate bond. This is demonstrated with an arrow. The diagram is missing an overall charge of + on the ammonium ion it produces. Coordinate bonds act the same as covalent bonds.
Once you have your covalent bonds, you need to know about covalent substances and their properties. There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these. Iodine (shown below) has a regular arrangement which makes it a crystalline substance and water, as ice, has a crystalline structure as well.

The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
The other kind of covalent substance you need to know is macromolecular. This includes giant covalent structures such as diamond or graphite, which are allotropes of carbon. Non-metallic elements and compounds usually form these crystalline structures with a regular arrangement of atoms.
Allotropes are different forms of the same element in the same physical state.
Diamond is the hardest naturally occurring substance on earth therefore is good for cutting glass and drilling and mining. It has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
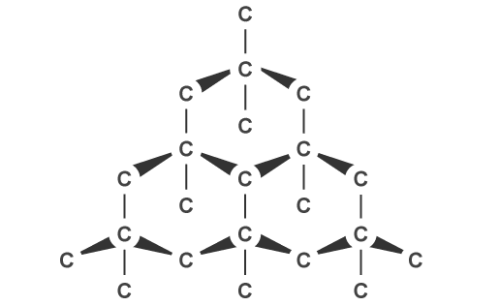
Graphite, on the other hand, can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.

Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
SUMMARY
Covalent bonding involves one or more shared pairs of electrons between two atoms. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms.
Dot and cross diagrams use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.
The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.
A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.
The formation of ammonium is an example of this.
There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these.
The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
Giant covalent structures such as diamond or graphite are allotropes of carbon. Allotropes are different forms of the same element in the same physical state.
Diamond has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
Graphite can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.
Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
Happy studying!
Unveiling Solar Innovator System: A Comprehensive Review
Introduction: Are you grappling with extreme weather conditions that force you to rely on power-hungry appliances throughout the day? The Solar Innovator System might be the game-changer you've been searching for. In this review, we'll explore the legitimacy and worthiness of the Solar Innovator System, a revolutionary digital product designed to create a nature-inspired 3-dimensional solar sphere.
What is the Solar Innovator System? The Solar Innovator System is a groundbreaking digital solution aimed at helping you construct a 3D solar sphere, inspired by nature. This innovative system claims to slash your electric bills by an impressive 50-70%, offering a reliable and eco-friendly energy source to power your household appliances.
Created by Mark Pierce, the Solar Innovator System is a systematic program that guides users through the process of building a home power plant using simple materials. This 3D Solar Sphere ensures energy independence even during nationwide blackouts, boasting portability and minimal maintenance requirements.
Advantages of Solar Innovator System:
Space-Efficient: The Solar Innovator System enables you to create a product that occupies less space than traditional solar panels. According to Pierce, it can be conveniently installed on balconies or near windows, making it accessible to everyone, regardless of engineering skills.
Power Output Surge: Mark Pierce claims that the Solar Innovator System equips users with knowledge to create a 3D solar sphere that boosts power output by up to 101%. The system is designed to efficiently capture sunlight and convert it into immediate, cheap, and eco-friendly power.
About Mark Pierce – The Innovator: Mark Pierce, the brains behind the Solar Innovator System, was inspired by a near-death experience during a power outage. This motivated him to research and create a sphere-shaped solar panel capable of generating more power than traditional flat-based panels.
How Does the Solar Innovator System Work? The Solar Innovator System is a comprehensive digital product comprising video guides, illustrations, and step-by-step instructions for building a reliable 3D solar sphere. This gadget aims to revolutionize renewable energy, providing users with a clean and dependable power source. The 3D solar sphere works by allowing photons to release electrons, generating an electric current and boasting multiple photovoltaic cells for increased efficiency.
Features and Benefits:
User-Friendly: Pierce emphasizes that the Solar Innovator System is user-friendly, offering over-the-shoulder video guides, illustrated blueprints, materials cheat sheets, and simple instructions for creating practical 3D spherical solar panels.
Pricing: Available for a limited time at $39, the Solar Innovator System provides manuals, videos, guides, and instructions through immediate email delivery. Prices are expected to rise in the future.
Refund Policy: With a 60-day money-back guarantee, Mark Pierce assures a refund for dissatisfied customers, underlining confidence in the product's effectiveness.
Noiseless and Safe: The Solar Innovator System produces zero noise pollution and promotes the generation of safe solar panels without emitting toxins or gases.
Conclusion: The Solar Innovator System presents a promising solution for those seeking to harness clean, renewable power through a 3D solar sphere. With thousands of satisfied users and a simple construction process, this digital product could potentially reduce your electric bills significantly. Act now to secure the current pricing and embark on your journey towards energy independence.